For Veterinaries
Some useful links:
Diagram for inheritance of PRA (Progressive Retinal Atrophy)
reproduced with kind permission from Swedish Siamese Society (SES)
PRA is a hereditary eye disorder where the retina slowly degenerates, making the cat blind. This page illustrates the inheritance.

= Normal - cat without the gene for PRA, does not pass on the gene for PRA

= Carrier - cat with one gene for the disorder, can pass this gene on to progeny, will not develop PRA

= Affected - cat with two genes for the disorder, will pass this gene on to progeny, will also develop PRA
________________________________________________________________________________
 |
+ |  |
Normal cat mated with normal cat
 |
 |
 |
 |
100% of the progeny will be normal/without the gene for PRA
_______________________________________________
 |
+ |  |
Normal cat mated with carrier
 |
 |
 |
 |
50% will be carriers, 50% will be normal
_______________________________________________
NB! The following combinations are not recommendable and should not be done
 |
+ |  |
Carrier mated with carrier
 |
 |
 |
 |
25% of the progeny will get PRA, 50% will be carriers and 25% will be normal
_______________________________________________
 |
+ |  |
Carrier mated with affected cat (has PRA)
 |
 |
 |
 |
50% of the progeny will be affected and 50% will be carriers
_______________________________________________
 |
+ |  |
Affected cat mated with normal cat
 |
 |
 |
 |
100% of the progeny will be carriers
_______________________________________________
 |
+ |  |
Affected cat mated with affected cat
 |
 |
 |
 |
100% of the progeny will be affected
_______________________________________________
Copyright © Svenska Siamessällskapet 2011
Bordetella bronchiseotica infection in cats
By Lies Klösters. 2007.
(Reprinted with permission.)
The widespread vaccination of cats against feline calici virus (FCV) and feline herpes virus (FHV) has failed to eliminate the problem of feline upper respiratory tract disease (URTD).
Bordetella bronchiseptica (Bb) has long been recognized as an important agent in canine kennel cough but only in recent years has its role in the development of URTD of cats been recognised. The clinical signs of Bb infection are very similar to those of respiratory virus infection and there is mounting evidence that infection is widespread.
Stress predisposes cats to the development of disease associated with Bb and this is most common in multicat households and catteries. The disease is most severe in kittens (where fatal bronchopneumonia has been reported). URTD raises important welfare issues and has significant financial implications for cattery owners and cat breeders. The development of a vaccine (Nobivac Bb for cats) for the protection of cats against this disease is an important breakthrough in URTD prevention.
Clinical signs of Bordetella bronchiseptica infections
Bordetella bronchiseptica associated upper respiratory tract disease (URTD) is a complex disease. There is a considerable overlap between the clinical signs seen with other agents that can cause URTD, including feline calici virus (FCV) and feline herpes virus (FHV).
In most cats the disease caused by Bordetella bronchiseptica (Bb) is mild and signs disappear after about 10 days. Life-threatening bronchopneumonia may develop particularly in young kittens.
In studies in cats where Bb is known to be the only causative agent, clinical signs of disease typically include fever, sneezing, nasal discharge, submandibular lymphadenopathy, and râles.
Coughing, although frequently reported in cats, does not seem to be as characteristic a feature as in Bb infection of dogs. Unlike most cases of infectious URTD in cats acute deaths occur, particularly in young kittens, when the disease progresses to bronchopneumonia.
Some cats may become long-term carriers and recovered cats have been shown to shed Bb for at least 19 weeks after initial exposure.
Diagnosis of Bordetella bronchiseptica infection in cats
Disease caused by Bordetella bronchiseptica (Bb) cannot be diagnosed solely by visual or physical examination.
Many of the clinical signs of Bordetella bronchiseptica infection in cats (with the exception of coughing) mimic those seen with other respiratory pathogens. Response to antibiotic treatment may provide limited supportive evidence of involvement of a bacterial pathogen in the disease.
Bb infection is best diagnosed from oro-pharyngeal swabs, or from swabs of nasal discharge of affected cats. Samples should be taken using sterile cotton wool swabs placed into charcoal transport medium before plating on to a selective medium such as Bordet-Gengou.
Chronic carrier cats often shed relatively few organisms and require repeated oro-pharyngeal culturing. Furthermore, isolation (as with viral respiratory pathogens) does not confirm that the bacterium is the causative agent of the URTD.
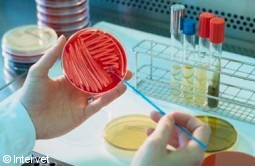
Bacteria from oro-pharyngeal swabs
are plated on selective medium.
Pathological effects of Bordetella bronchiseptica
The inflammatory response to Bordetella bronchiseptica infection is initiated by damage to the respiratory epithelium causing the release of inflammatory cytokines.
The release of toxins following colonisation is responsible for local and systemic inflammatory damage for the first 3-5 days after infection. The first clinical signs of Bordetella infections may be noticed after this time. After onset of the local immune response the bacteria are gradually eliminated (Bemis et al 1977). In cats most illness appears self-limiting with spontaneous resolution occurring after about 10-14 days. However, severe bronchopneumonia associated with Bordetella bronchiseptica (Bb) may occur, particularly in kittens.
Bb is adapted to establish long-term asymptomatic infection. The bacterium is able to modulate the immune response associated with infection (Yuk et al 2000). Infections are typically chronic, often asymptomatic, and notoriously difficult to clear even with antibiotic therapy. There is increasing evidence of resistance to certain antibiotics including tetracyclines and ampicillin.
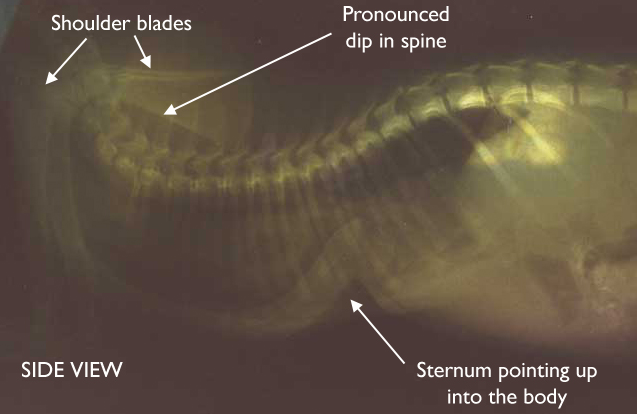
Thoracic radiograph of a cat with bacterial bronchopneumonia
(lateral view). Widespread patchy areas of alveolar pulmonary
infiltrate and diffuse bronchial pattern.
Treatment of Bordetella bronchiseptica infection in cats
Apart from general measures, antibiotic therapy should be considered for the treatment of upper respiratory tract disease (URTD) in cats.
Cleaning ocular and nasal discharges from cats with Bordetella bronchiseptica infection is an important nursing consideration.
Congestion and malaise combine to cause inappetance so fluid therapy and nutritional support are vital. Ocular and nasal discharges should be cleaned away regularly and steam inhalation may help to reduce congestion.
Antibiotic therapy is always indicated with bacterial URTD. Antibiotics should be continued for as long as discharges are present. Recent studies have shown that isolates of Bordetella bronchiseptica (Bb) from cats are susceptible to tetracycline and doxycycline but that resistance to trimethoprim and ampicillin is widespread.
Infection can be treated with
- tetracycline (10mg/kg PO q8h)
- doxycycline (10mg/kg PO q24h)
- amoxicillin/ clavulonic acid (62.5mg/cat PO q12h).
Some recent isolates of Bb from cats show evidence of development of tetracycline-resistance.
Prevention and control of Bordetella bronchiseptica infection in cats
Until now prevention of feline upper respiratory tract disease (URTD) has been limited to cattery management.
Good Cattery Practice (particularly good hygiene practices and the reduction of stress in cats) is an important aid in the prevention of URTD in cats. Bb does not survive long outside the cat and is killed by many common disinfectants so routine hygiene measures are sufficient to prevent disease spreading through the environment. Removal of chronically-infected cats from affected catteries can also be considered.
An intranasal vaccine (Nobivac® Bb for cats) is now available in some countries and this can be used to help prevent URTD outbreaks in catteries. Vaccination is also indicated to help prevent respiratory disease in cats which:
- come into contact with other cats and dogs
- are boarded at catteries or are exhibited.
Cat vaccination programmes should always be combined with good husbandry: good nutrition, sanitation, ventilation, parasite control and control of other respiratory pathogens to minimise the occurrence of clinical disease.
During parturition, queens may pass Bordetella bronchiseptica to their offspring; kittens obtained from breeding catteries and rescue shelters are, therefore, particularly at risk from this disease. Since Bordetella bronchiseptica infection in kittens can cause acute death as a result of bronchopneumonia, this risk must be taken seriously. The potential for mortality and the general morbidity caused by infectious URTD warrants the use of measures designed to tackle disease, particularly in high-risk animals. The use of Nobivac® Bb for cats is an important part of such preventative schemes.
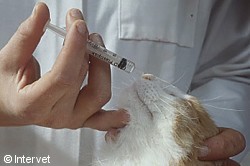
Intranasal vaccination with
Nobivac® Bb for cats.
Author's Notes:
- The information in this article was copied from the NobivacBb website. For more reading about this topic, visit www.nobivacbb.com. I sincerely want to thank Intervet for allowing us to use their information for this article.
- When this article was written, the vaccine against Bordetella bronchiseptica in cats was not available in every country. Please contact your vet to discuss the possibilities available to you.
An Overview of Feline Enteric Coronavirus and Infectious Peritonitis Virus Infections
By Niels C. Pedersen, 1995
Department of Medicine and Epidemiology
School of Veterinary Medicine
University of California
Davis, California 95616
(Reprinted with permission.)
Introduction
The purposes of this discussion were to review past knowledge about feline enteric coronavirus (FECV) and feline infectious peritonitis virus (FIPV) infections and to update this knowledge with information gleaned from the International Workshop on Feline Enteric Coronavirus and Feline Infectious Peritonitis held at the University of California, Davis, on August 12-14, 1994. Much of the information presented at this workshop was either entirely new or has been reported only in approximately the last three years. Taken as a whole, this new information provided us with a clearer picture of these infections than previously possible. We now have a much better understanding of the evolution of feline coronaviruses (FCoVs), the probable origin of FIPV as a mutation of FECV, the nature of the FECV carrier state, and control methods with which FIP losses can be decreased.
Historical Perspectives
Background
Feline infectious peritonitis (FIP) was first described as a specific disease entity of cats more than 30 years ago (Holzworth, 1963). Cases of FIP have been subsequently reported from virtually every country of the world. Wolfe and Griesemer (1966) were the first to propose a viral etiology for FIP. Zook and coworkers (1968) confirmed that the causative agent was indeed a virus, and Ward (1970) identified the causative agent as belonging to the coronavirus family. The discovery that FIP virus (FIPV) caused two distinct forms of disease, (i.e., wet, or effusive, and dry, noneffusive), was reported by Montali and Strandberg (1972). The close antigenic relationship of the FIP virus (FIPV) to canine, porcine, and human coronaviruses was reported in 1978 (Pedersen et al. 1978).
FIP was originally thought to be an uncommon and fatal disease manifestation of a single ubiquitous coronavirus (Pedersen, 1976b). However, this was disproved with the discovery of the feline enteric coronavirus (FECV)(Pedersen et al, 1981). Although FECV was found to be morphologically and antigenically indistinguishable from FIPV, it became apparent upon animal inoculation studies that FIPV and FECV were two distinct entities in terms of biologic behavior (Pedersen et al, 1981). Therefore, a single virus was not responsible for both the large proportion of healthy coronavirus seropositive cats and the small proportion of animals with FIP. The hypothesis was then put forward that FIPV was merely a simple mutation of FECV that occurred frequently during the course of FECV infection (Pedersen and Floyd, 1985).
Is FIP a recent disease of cats?
FIP was not observed before the 1950s, even in institutions like the Angell Memorial Animal Center in Boston where careful postmortem examinations of all diseased cats have been routinely conducted for decades (Holzworth, 1963). This indicates that FIP is either a relatively new condition of cats or that FIPV has been present for ages, but there have been changes in the way modern cats are reared and housed that have favored the infection and disease.
Evidence favoring environmental changes as the main cofactor behind rising FIP mortality is most compelling. The period following World War II to present has seen a dramatic shift in human demographics from a rural to urban/suburban setting. During this same period the pure breeding of cats became popular and the domestic cat progressively replaced the dog as the most common household pet (there are more than 60 million cats in homes in the United States today compared to less than 50 million dogs). With the emergence of the cat as an important household pet, the numbers of indoor environments harboring large numbers of cats has also increased. The breeding of purebred cats has burgeoned during this same period. With increasing pet cat ownership, pounds became inundated with progressively larger numbers of cats. In an attempt to save as many unwanted pet cats as possible, many large, privately financed and operated shelters for cats came into existence (Postorino Reeves, 1995). The feral cat population has also increased in relation to the population of owned cats. Semi-managed colonies of feral cats are now common in many neighborhoods.
Nomenclature of Feline Coronaviruses
There are two recognized coronaviruses of cats, FECV and FIPV. Because FECV and FIPV are virtually the same virus in genetic and antigenic terms, a generic name "feline coronavirus" (FCoV) is often used to encompass both agents (Addie et al, 1995; Vennema et al, 1995). All of these different names can lead to some confusion. Therefore, as a rule, FCoV should be used only in situations where genetic similarities or biological distinctions between FIPVs and FECVs either are not important or cannot be made. For instance, because serologic tests cannot distinguish FECVs from FIPVs, it is more correct to talk about "FCoV serology" rather than "FIPV serology" (Pedersen, 1995). In contrast, when referring to natural or experimental infections with these two agents, it is more appropriate to talk about the precise type of virus, as in FECV (enteritis-inducing) or FIPV (FIP-inducing). The terms virulent and avirulent FCoVs have also been used as synonyms for FIPVs and FECVs, respectively (Addie et al, 1995).
The Relationship of FIPV and FECV
FIPV is morphologically and antigenically indistinguishable from the ubiquitous feline enteric coronavirus (FECV) (Boyle et al, 1984: Pedersen et al, 1981). Both viruses also co-exist in the same environments and sporadic cases of FIP have followed naturally and experimentally induced outbreaks of FECV infection in closed, pathogenfree cat populations by several months or more (Hickman et al, 1995; Vennema et al, 1995). Observation like this have led some to conclude that FIPV is a simple mutant of FECV (Pedersen and Floyd, 1985; Pedersen, 1988, 1991; Vennema et al, 1995). Indeed, the genomes of FIP-inducing viruses isolated from focal outbreaks have been virtually identical to the endemic or infecting strain of FECV, but significantly different from FECVs and FIPVs isolated from geographically distinct premises (Vennema et al, 1995; Pedersen and Vennema, University of California, Davis, personal observation, 1994). The ease with which FECV can mutate is supported by experiences with mutability in related coronaviruses of mice and pigs (Horzinek et al. 1995). Because FIPVs and FECVs are virtually identical, except for biologic disease potential, FECV and FIPV have been referred to as biotypes of Serotype I or II FCoVs (Vennema etal, 1995).
Although there is no doubt that FIPVs are simple mutants of FECVs, the exact mutation responsible for the change in disease potential has yet to be discovered (Vennema et al, 1995). Furthermore, the origin of the mutation in regards to the cat that actually develops FIP is unknown. One possibility is that FIP-inducing variants are generated at a low level during primary or secondary infections with FECV and cause disease in the same cat in which they originated. A second possibility is that the mutant virus is generated in the intestine of one FECV-infected cat, shed in the feces, and then inadvertently ingested by other susceptible animals.
Serotypes of FIPV and FECV
All FCoVs, whether they are FECVs or FIPVs, belong to two distinct serotypes, called Type I and Type II (Pedersen et al, 1984). Serotype I FCoVs comprise 70 to 95% or more of FIPV and FECV isolates throughout the world (Hohdatsu et al, 1992). Serotype I FCoVs are very difficult to grow in cell culture. When propagated, they grow preferentially on Felis catus, whole fetus-4 (Fcwf-4) cells rather than Crandell feline kidney (CrFK) cells and are not neutralized by antiserum to canine coronavirus (CCV). Serotype II FCoVs are more readily propagated in cell culture, including growth on CrFK cells, and they are neutralized to high titer by CCV antiserum. Therefore, Serotype II FCoVs are more CCV-like than Serotype I FCoVs. Examples of Serotype I FCoVs include all but one of the present FIPV-UCD isolates (FIPV-UCD1-5 and 8-10), FECV-UCD, FECV-RM and FIPV-TN406 (Vennema et al, 1995). Serotype II FCoVs include FIPV-79-1146, FIPV-Norl5, FIPV-DF2, FIPV-UCD6, and FECV-79-1683.
The Relationship of FCoVs to Coronaviruses of Other Species
The role of other species of animals in FECV infection of cats is unknown. Coronaviruses of swine, rodents, birds, and man appear to be too distantly related to FCoVs to infect cats. However, from 5 to 30% of the feline coronavirus isolates, depending on the area of the world, are distinctly CCV-like in their structure (Hohdatsu et al, 1992). One CCV strain has induced mild enteritis in laboratory cats, while a second CCV isolate has induced FIP when experimentally inoculated into cats (McArdle et at, 1992). Therefore, some strains of CCVs may infect cats directly; however, such direct infection is probably uncommon; genomic mapping studies indicate that Serotype II FCoVs are actually recombinants between Serotype I FCoVs and CCV that occurred some time in the past (Vennema et at. 1995).
Epidemiologic Considerations
FECV Infection
FECV is endemic in virtually all environments in which large numbers of cats are kept closely confined, and where litter, food, and water containers are shared. About one-fourth of animals from single cat households will have FCoV antibodies, while from 75 to 100% of cats from pure breed catteries are seropositive at any given time (Pedersen, 1976). The presence of large numbers of kittens, or the frequent movement of new cats in and out of the environment, will further enhance the likelihood of infection.
Susceptible cats introduced into FECV endemic environments are infected almost immediately, with seroconversion in 7 to 14 days (Pedersen et al, 1981). Kittens born to queens within endemic households become susceptible to infection when they lose their passive systemic and local (lactogenic) maternal immunity, usually from 6 to 10 weeks of age.
FIPV Infection
FIP is mainly a disease of younger cats; death losses begin to appear shortly after weaning and peak at 6 to 18 months of age (Kass and Dent, 1995; Pedersen, 1976a, 1983a, 1988, 1991). Mortality decreases thereafter, with relatively few cases occurring after 3 to 5 years of age.
Up to three-fourths of the cases of FIP in the USA occur among cats raised in pedigreed catteries and large multiple-cat households or shelters. The proportion of FIP cases in pet cats from one- or two-cat homes, whether indoor or indoor/outdoor roaming, is low. This differential is not as evident in some northern European countries such as Switzerland (Fehr et al, 1995). The proportion of pet cats affected with FIP is greater and mortality often appears in late summer when the weather becomes colder and cats start spending more time together indoors.
The overall mortality to FIP among pet cat households consisting of one or two older cats is on the order of 1:5,000, while mortalities of around 5% are common in densely housed groups of cats (Addie et at. 1995; Fehr et at. 1995; Postorino Reeves, 1995). Outbreaks of FIP within high risk populations usually take the endemic form, with clustering of one or more cases within a few months followed by periods where no cases are seen. Epidemics of the disease are less common, appearing explosively and involving as high as 40% or more of the younger cats. Following such epidemics, the disease rapidly returns to its endemic form.
Although virtually all large groups of group-housed cats suffer from endemic feline coronavirus infection, only about one-third of such groups will report FIP losses (Addie et al, 1995; Kass and Dent, 1995). There are several explanations for this paradox. First, many kitten deaths go undiagnosed for lack of pathologic examination. Second, husbandry practices vary greatly from cattery to cattery, and some husbandry practices are more favorable to FECV replication and therefore to FIP. Third, there are numerous strains of FIPV and each may vary in its ability to induce FIP (Pedersen et at. 1984). Fourth, there are many cofactors that influence the actual incidence of FIP in addition to mere exposure to the virus.
Numerous extraneous influences, termed cofactors, may affect the incidence of FIP. The incidence of FIP is not affected by the gender of the cat. A breed predisposition has also not been confirmed, although certain bloodlines and matings within a breed may be at greater risk. The incidence of FIP among purebred catteries increases in proportion to the number of animals kept in the same facility (Kass and Dent, 1995). Littermates of affected animals are several times more likely to also develop FIP than nonlittermates, undoubtedly because siblings are genetically similar, exposed in the same manner, and share similar cofactors. Stress of various types also appear to play an important role in FIP. These stresses include:
- pregnancy in younger queens,
- elective surgical procedures such as spays, castrations, and declaws in younger cats,
- the frequently combined stresses of weaning, sale, shipment, and adaptation to new homes, and
- intercurrent infectious diseases.
Stress is most likely to have a deleterious effect on disease outcome if it occurs at the initial stages of infection. For kittens raised in FCoV endemic households, this initial infection occurs between 6 and 10 weeks of age, just when stresses of all types are greatest. Furthermore, this is also a period in the development of the cat's immune apparatus when it is difficult to generate protective FIPV immunity to vaccination, and presumably, natural infection as well (J Gerber, Pfizer Animal Health, Inc., Lincoln, NE, personal communication, 1994).
Feline leukemia virus (FeLV) infection was once a major potentiator of FIP, being found in 30 to 50% of all cats with naturally acquired disease (Cotter et al, 1973; Pedersen, 1976). It has been shown experimentally, that if an otherwise healthy cat has an immunologically contained focus of FIPV infection in its body, a subsequent FeLV infection will interfere with that immunity and cause the infection to become active (Pedersen and Floyd, 1985). With the virtual elimination of FeLV from catteries and other multiple-cat households, the linkage between FIP and FeLV has become uncommon. Fewer than 5% of cats with FIP are currently FeLV positive (Pedersen, 1991), and those with dual infections are more likely to be from pet households than from catteries. Although the incidence of FeLV infection has drastically decreased in the same households that are at risk for FIP, the incidence of FIP has not decreased proportionally.
Pathogenesis of Feline Coronavirus Infections
FECV Infection
FECV is spread by the fecal-oral route, and like all fecal-oral pathogens, certain husbandry factors favor both maintenance and transmission of the agent. The three essential ingredients being:
- a reservoir of infected animals to serve as a source of virus within the household,
- a continuous source of susceptible animals, and
- unfavorable husbandry practices that facilitate fecal contamination of the environment and fecal-oral transmission.
The actual reservoir for the virus is probably the small intestine; virus shedders being cats that are either undergoing or recovering from primary or secondary infections.The carrier state that follows these initial or recurrent infections appears to be brief in many animals, lasting only for a few weeks or months. Virus produced in the intestinal tract is shed in the feces. Virus particles can probably survive for several weeks in dried feces, in litter, and in porous flooring thus providing an additional reservoir for infection.
The source of susceptible animals is threefold:
- cats raised in the cattery 6 to 10 weeks of age or older (after they have lost their maternal immunity),
- susceptible cats brought into the cattery or household from the outside, and
- previously infected cats that have lost their immunity with time.
These susceptible animals act as fuel for the infection — the more susceptible cats that are added to the environment, the greater the severity of infection (Kass and Dent, 1995). Improper husbandry practices that assist in the transmission of virus from the feces of infected cats to the mouths of susceptible animals, as well as situations that suppress the ability of the cat to combat the infection, act to enhance the spread and severity of infection.
Following ingestion, the virus travels through the digestive tract to the small intestine and binds itself to the surface of the epithelial cells (enterocytes) that line the tips of the intestinal villi. It will then enter the enterocyte, rapidly replicate in the cytoplasm, and kill the host cell. Virus particles released from the dying enterocytes then attach themselves to other noninfected enterocytes in the vicinity and the cycle will continue. If sufficient numbers of gut cells can be destroyed before immunity takes place, clinical signs of enteritis will occur.
FIPV Infection
FIP occurs when a cat is exposed to variants of FECVs that have mutated and gained the ability to replicate in macrophages. This ability to replicate in macrophages is the essential difference between FECVs and FIPVs (Stoddart and Scott, 1989). Once FIPV begins replicating in macrophages, it becomes a systemic intracellular pathogen; macrophages are wandering cells and can easily spread the virus to other tissues and organs of the body.
The series of events that frequently ends in the death of the host cat from FIP are set into motion when the virus is ingested by a macrophage, often in the mesenteric lymph nodes. FIPV replicates rapidly within the cytoplasm of the macrophage; the cell then ruptures and releases a myriad of new virions into the surrounding spaces. These particles are rapidly taken up by more macrophages, and the rate of virus replication escalates in a logarithmic manner. Some of the infected macrophages escape from the mesenteric lymph nodes and migrate in the blood to venules in the serosal and pleural surfaces of the abdominal and thoracic viscera, the meninges and ependyma of the brain and spine, and the uveal tissues of the eyes. The infected macrophages then exit the venules where they continue to replicate virus. In response to this virus replication, more macrophages are recruited from the blood and enter the area.
The characteristic inflammation of FIP results from both the effects of the virus on macrophages and the effects of the host's own immunity on the infected cells. Because infected macrophages tend to congregate around small venules in the target tissues, the classical lesion of FIP is vascular inflammation or "vasculitis."
Clinical Features of Feline Coronavirus Infections
FECV Infection
FECV infection is usually inapparent, although in severe cases there may be transient vomiting followed by a mild to severe diarrhea lasting several days at most (Dea et al, 1982; Hickman et al, 1995; Pedersen et al, 1981). Lethal FECV infections are very uncommon (McKeirnan et al, 1981).
FIPV Infection
Two forms of FIP occur in nature. The classical, effusive, or wet form of the disease is the most common and the easiest to diagnose. Wet FIP targets the body cavities, in particular the abdomen (Table 1), and the characteristic fluid effusions are easily identified. The less common noneffusive or dry form of FIP while still centered around the abdomen, involves a wider range of organs, including the eyes and central nervous system (CNS) (Table 1); however, the fluid effusions are absent or minimal, thus removing a major diagnostic feature.
| TABLE 1 Variability In Sites of Gross Lesions in Effusive and Noneffusive FIP |
||||||
| Clinical Signs Referable to involvement of the: |
|
|||||
| Peritoneal cavity | 62/107=58% | 30/94=32% | ||||
| Peritoneal and pleural cavities | 24/107=22% | 4/94=4% | ||||
| Pleural cavity | 12/107=11% | 1/94=1% | ||||
| Peritoneal cavity and eyes | 3/107=3% | 7/94=7% | ||||
| Peritoneal cavity and CNS | 2/107=2% | 0/94=0% | ||||
| Peritoneal and pleural cavities and CNS | 1/107=1% | 3/94=3% | ||||
| Peritoneal and pleural cavities and eyes | 1/107=1% | 2/94=2% | ||||
| Peritoneal cavity and CNS | 1/107=1% | 0/94=0% | ||||
| Peritoneal cavity, CNS, eyes | 1/107=1% | 2/94=2% | ||||
| Pleural cavity, CNS, eyes | 0/107=0% | 1/94=1% | ||||
| CNS | 0/107=0% | 22/94=23% | ||||
| Eyes | 0/107=0% | 14/94=15% | ||||
| CNS and eyes | 0/107=0% | 8/94=9% | ||||
The clinical course of FIP ranges from a few days to several months or more, with wet FIP being more rapidly progressive than the dry form. At the time of death or euthanasia, cats will usually have one form or the other; however, in the entire course of illness the form of the disease often changes. Most cases with dry FIP begin with a brief and often undetected bout of wet FIP. It is also not unusual for cats to suffer from the dry FIP for weeks or months before developing wet FIP shortly before death. This change from dry to wet FIP is associated with the eventual collapse of the immune system because of the relentless effect of the infection on the body. With the total collapse of cell-mediated immunity, the level of virus replication increases dramatically and the lesions become much more widely disseminated and change in form from granulomatous (dry) to pyogranulomatous (wet).
There are a number of clinical signs that are shared between wet and dry FIP, and some features that are distinct. Common features include a chronic, fluctuating, antibiotic unresponsive fever, progressive anorexia, and weight loss. Stunting of growth is a common abnormality in kittens with FIP and may precede other signs by many weeks or months. Cats with FIP also tend to show progressive rises in their serum total proteins and globulins, an elevated white blood cell count with high levels of polymorphonuclear neutrophils and low levels of lymphocytes, and a mild to moderate anemia of the "chronic disease type". The serum and urine often become a dark yellow because of bilirubin staining. More specific clinical signs are also observed depending on the region of the body that is most affected and the form of the disease. Peritonitis with the accumulation of large amounts of fluid occurs in 75% or more of cats with wet FIP (Table 1). Pleuritis with accumulations of fluid in the chest is seen in about 25% of cats with effusive FIP. Abdominal fluid, or ascites, is manifested by a distended abdomen. If the cat is an intact male, the scrotum may appear enlarged. The tissues surrounding the testicles are extensions of the same tissue that lines the peritoneal cavity and peritoneal organs. Cats with chest fluid often have difficulty breathing.
Although the abdomen is still the most common site to find lesions in cats with dry FIP (Table 1), ascites is absent or minimal. Instead, there are large surface-oriented granulomas in the mesenteric lymph nodes, kidneys, and less commonly the liver or cecum. Granulomatous lesions are frequently palpable through the body wall or visible on ultrasonography.
Just as ascites or pleural effusions are typical of wet FIP, gross and clinical signs referable to the eyes and CNS are observed almost exclusively in cats with dry FIP (Table 1). Ocular signs are often manifested by a cloudiness in the fluid behind the cornea, inflammatory precipitates on the back of the cornea, an irregularly shaped pupil, or even a change in eye color (because of inflammation in the iris). Nervous system signs are varied, again depending on the structures that are affected. Weakness or paralysis of the hind limbs is probably the most common neurological sign, associated with spinal cord lesions. Paralysis of the major nerves to the front limbs and the head and neck have also been seen. Involvement of the brain can cause dementia, personality changes (withdrawal, rage, hiding), convulsions, head-tilts, or compulsive circling.
Why are lesions in effusive FIP concentrated in the core of the body (i.e.. abdomen, chest cavity), while lesions of noneffusive FIP are concentrated in the nervous system (the eyes are part of the nervous system)? The explanation again lies with the intensity of the host immunity. The nervous system has a special barrier (blood-brain barrier) that prevents the access of noxious substances from the rest of the body. Unfortunately, the blood-brain barrier also impedes the influx of immune cells and antibodies from the core of the body. Therefore, immunity must be very intense in the core of the body before significant levels of immunity can spill over into the nervous tissues. In cats with noneffusive FIP, the immune response to the virus is partial at best, and partial immunity will be most beneficial within regions of the body where the host has ready access, which is the core of the body.
Clinicopathologic Features of Feline Coronavirus Infections
FECV Infection
There are no characteristic clinicopathologic features for FECV infection.
FIPV Infection
Complete blood counts tend to show similar abnormalities, regardless of the form of disease. An elevated white blood cell count (leukocytosis) associated with increased numbers of polymorphonuclear neutrophils (pus cells) and decreased numbers of lymphocytes (immune cells) is seen in most affected animals.
A mild to moderate anemia of the nonregenerative form is also common, especially among cats that have been ill for some time. The anemia is the type that is often associated with chronic illness, regardless of cause.
The total protein levels in the blood are often elevated, mainly because of an increase in the levels of antibody globulins and a decrease in albumin. Fractionation of abnormal sera by starch gel electrophoresis demonstrates elevations in both inflammatory (alpha) and antibody (beta and gamma) globulins. The decrease in albumin is proportional to the anemia.
The serum is often yellow tinged because of elevated levels of bilirubin pigment (hyperbilirubinemia); the urine has similar changes (hyperbilirubinuria). The degree of hyperbilirubinemia and hyperbilirubinuria are often out of proportion to clinical icterus (yellowing of the tissues of the body), a differentiating feature from other types of liver disease such as hepatic lipidosis and chronic biliary cirrhosis.
Fluid from the abdomen or chest of cats with wet FIP is fairly characteristic. It is yellowish in color, has a mucinous, sticky or egg-white consistency, and contains numerous polymorphonuclear neutrophils and monocyte/macrophages. The total protein levels in the fluid are often from 75 to 100% of serum levels. Abdominal fluid is not visibly bloody; however, chest fluid is frequently bloodtinged and the yellowish color may not be appreciated until the cells are centrifuged or allowed to settle to the bottom of the collection tube. Cerebrospirial fluid from cats with extensive meningeal or ependymal involvement often contains elevated levels of protein and neutrophils. Synovial fluid, especially from cats with acute effusive FIP, may be inflammatory in nature with increased numbers of neutrophils.
Aspirations (or sometimes biopsies) of lesions on abdominal organs of cats with dry FIP will show a mixed inflammatory infiltrate, including nondegenerative neutrophils, monocyte/macrophages, plasma cells, and lymphocytes.
Pathologic Features of Feline Coronavirus Infection
FECV Infection
The only detailed pathologic description of FECV infection has been provided by Pedersen and co-workers (1981). The typical lesion consists of a transient blunting of the small intestinal villi associated with a loss of the mature apical epithelium. This is followed by hyperplasia of the crypt epithelium and regeneration of normal villus architecture.
FIPV Infection
The gross and microscopic lesions of FIP have been amply described (Holmberg and Gribble, 1973; Montali and Strandberg, 1972; Wolfe and Griesemer, 1971). It is strongly recommended that all suspected cases of FIP be confirmed either by a partial or complete postmortem examination. A partial examination would be to visually confirm the nature and location of the lesions within the body. In addition to the characteristic fluid, cats with wet FIP will have a diffuse inflammation of their peritoneal and/or pleural linings. The peritoneal surfaces frequently have a "ground glass" appearance and the omentum is usually highly edematous. Cats with dry FIP will demonstrate several large surface-oriented granulomatous lesions in certain target organs, especially the kidneys, mesenteric lymph nodes, liver, meninges, and ependyma.
Examination of the lesions under the microscope will add another level of accuracy. Small, diffuse, peritoneal/pleural surface-oriented pyogranulomatous lesions (focal accumulations of macrophages with a rim of polymorphonuclear neutrophils) are typical of wet FIP. Larger, more focal, surface-oriented granulomatous lesions, consisting of a central accumulation of macrophages with dense surrounding infiltrates of plasma cells and lymphocytes extending downward into the parenchyma of the affected organs are typical of dry FIP. The presence of typically gross appearing lesions in characteristic sites, coupled with other historical and laboratory information, should be sufficient to make the diagnosis.
A major problem encountered with postmortem diagnosis is the reluctance of pathologists to make a definitive diagnosis of FIP without actually testing the tissues for the presence of the FIPV, with the rationale being that other types of pathogenic microbes may cause similar lesions. Unfortunately, tests to identify the FIPV in lesions are expensive and somewhat sophisticated, and are therefore rarely done. Nonetheless, if the pathologist states that the lesions are compatible with FIP in their location and appearance, and the historical and clinical information are also highly suggestive, it is extremely unlikely that some other disease agent is involved. Other infectious disease agents that could cause similar lesions are far less common than FIP, usually have different histories, and tend to cause distinctive, gross, and microscopic changes.
The Diagnosis of Feline Coronavirus Infections
Feline Enteric Coronavirus
It is impossible to diagnose FECV infection based on clinical signs. Most FECV infections are clinically inapparent. When gastrointestinal signs are seen, they cannot be distinguished from those caused by a myriad of other infectious and noninfectious causes.
A positive FCoV titer in a healthy cat is highly indicative of previous FECV exposure (Pedersen, 1995). Cats with higher FCoV antibody titers ( ≥ 1:100) are more likely to be shedding virus in their feces than cats with negative or very low (≤ 1:25) titers (Addie and Jarrett, 1995; Hickman et al, 1995: Pedersen et al, 1981).
FECV shedding in feces can be detected by electron microscopy on negatively stained fecal extracts (Dea et al, 1982; Pedersen et al, 1981). However, this procedure is cumbersome, expensive, and not routinely available. The PCR has been recently used to detect FECV RNA in feces (Herrewegh et al, 1995). However, this technique has not been validated, i.e., fecal PCR positive animals have not been confirmed to be infectious and PCR negative cats have not been shown to be noninfectious.
Feline Infectious Peritonitis
FIP, whether wet or dry in form, has many characteristic features (Table 2) and is not nearly as hard to diagnose as many would believe. The trick to making the diagnosis is to combine as many typical historical, physical, and clinicopathologic findings for FIP as possible. As an example, the more of the following historical, physical, and laboratory findings that the cat has, the more likely that it will have FIP; some clinical features will obviously carry more weight (significance) than others (Table 2).
| TABLE 2 Historical, Clinical, and Laboratory Findings in a Sick Cat and Their Relative Importance (Weighting) in the Diagnosis of FIP. |
||
| Historical Findings | Points | |
| 1. | Antibiotic-resistant, persistent, spiking fever | 10 |
| 2. | Abdominal effusion | 10 |
| 3. | Pleural effusion | 5 |
| 4. | Above effusions yellowish, mucinous high protein, fibrin tags, moderate numbers macrophages and PMNs | 15x signs 2 or 3 |
| 5. | Icteric serum | 5 |
| 6. | Palpable or visual masses in mes. LNs. kidney, ileo-cecocolic area | 5 |
| 7. | Aspirates or biopsy of above masses showing granulomatous inflammation | 15x sign 6 |
| 8. | Neurological abnormalities | 15 |
| 9. | Anterior uveitis/retinitis | 10 |
| 10. | Anterior uveitis with keratic precipitates | 25 |
| 11. | Characteristic complete blood count | 10 |
| 12. | Elevated serum globulin | 10 |
| 13. | Characterstic serum electrophoresis | 15 |
| 14. | FCoV antibody titer negative to 1:25 | 0 |
| 15. | FCoV antibody titer 1:100-1:400 | 5 |
| 16. | FCoV antibody titer 1:1600 | 10 |
| 17. | FCoV antibody titer 1:3200 or greater | 20 |
| Total the above points and multiply by the following: | ||
| 18. | The cat comes from a pure breed cattery or from a large multiple cat environment (pound, shelter, pet store, pet household, etc.) | 3x total pointsb |
| 19. | The cat is from 3 months to 3 years of age | 2x total pointsb |
| Cats scoring less than 75 are unlikely to have FIP; 75 to 200, FIP should definitely be considered among differential causes; greater than 200, FIP should be at the top of diagnostic list. b Weighting of other clinical signs are greatly influenced by the environment from which the cat originated and the cat's age at onset of illness. |
||
Because FIP is usually fatal, the term "nails in the diagnostic coffin" is appropriate in discussions of this type. Each of the above findings represents a different number of nails. In order to nail the lid down tightly — to make the diagnosis — a certain number of nails is required. Although this listing of signs and clinical findings and their relative significance is meant only as an example, the issue remains, that FIP cannot be diagnosed by any single finding. Moreover, in the scheme of things, findings such as a positive coronavirus antibody titer (especially in a healthy cat) are far less important, for instance, than the environment from which the cat came or a history of persistent antibiotic unresponsive fever. Also, the likelihood of one abnormality being related to another should also be considered. For example, neurological signs in a aged cat are unlikely to be caused by FIP, because FIP is uncommon in older animals; however, neurological signs in a younger cat, especially if it is from a cattery or other such environment, are very likely to be caused by FIP. Likewise, there are many causes of abdominal fluid in a cat, but hardly any other disease has fluid with the characteristic abnormalities found in cats with FIP.
Diagnosis of FIP by serologic means is virtually impossible because available antibody detection tests cannot distinguish antibodies generated by FECV exposure from those induced by FIPV infection (Pedersen, 1995). Although cats with FIP, especially those with the noneffusive form, tend to have antibody titers that are higher than those observed in healthy FECV exposed cats, the overlap is considerable. Therefore, it is considered veterinary malpractice to diagnose FIP in a cat by serologic means alone. Readers are referred to detailed descriptions published in this issue of Feline Practice on the interpretation and potential pitfalls of serology as a means to diagnose FIP (Pedersen, 1995; Richards, 1995).
Nucleic acid probes (Martinez and Weiss, 1993) and polymerase chain reaction (PCR) (Herrewegh et al, 1995) have been recently applied to FIP diagnosis. These tests can detect extremely low levels of viral RNA in tissues and various body fluids. The specificity of such tests for FIP diagnosis is based on two assumptions: 1) that FIPV is found within tissues, while FECV is found only in the feces, and 2) that it is possible to differentiate FIPV from FECV by PCRs based on the genomic maps of known strains of FECV and FIPV (Martinez and Weiss, 1993). Unfortunately, both assumptions may be incorrect. FCoV genome has been recently detected by PCR in the blood of many healthy cats in FCoV endemic households (Herrewegh et al, 1995). Therefore, it appears that FECV genome can also be present in tissues as well as feces, at least at some stages of infection. This would not be a problem if the PCR was specific for FIPV and not FECV. In order to make the PCR FIPV specific, PCR primers have been designed from regions of the genome that are presumably FIPV specific. These genomic maps have been obtained from so-called prototypic strains of FECV or FIPV, i.e., FECV-79-1683 and FIPV-79-1146; however, virtually all of the genetic differences between these two prototypic strains are caused by geographic variation rather than differences in disease potential (Vennema et al, 1995). Moreover, PCRs utilizing the genomes of the FECV-79-1683 and FIPV-79-1146 strains may preferentially detect biotype II rather than biotype I FCoVs (Vennema et al, 1995). Biotype II FCoVs constitute only 5 to 30% of field isolates of FIPV. Because of uncertainties regarding PCR for FIP diagnosis, it is strongly recommended that this procedure not be used for determining whether a cat has FIP.
Treatment of Feline Coronavirus Infections
FECV Infection
FECV infection is usually transient and clinically mild or inapparent. Treatment in such cases is not indicated; however, if the vomiting and/or diarrhea are severe, supportive treatment including nothing by mouth and fluid/electrolyte replacement may be needed for several days.
FIPV Infection
There is currently no effective way to cure a cat of FIP once the disease becomes clinically apparent; it is more than 95% fatal at this point. That is not to say that all cats infected with FIPV in nature die, because recovery in nature may be more common than death. However, cats that recover following natural exposure usually do so without ever having been noticeably ill. Therefore, the cats that present to owners and their veterinarians with overt signs of FIP are those animals that have already lost the battle.
Because FIPV is a viral disease, antibiotics are totally without effect and are not indicated. Certain antiviral drugs have been tried, and though they are effective in the test tube, they do not help the cat (Weiss, 1995). Anti-inflammatory and immunosuppressive drugs, such as glucocorticoids or cyclophosphamide, have been widely used to treat cats with FIP (Pedersen, 1976a). However, they will only delay the inevitable and have no curative powers in their own right. Immunostimulants of several types have also been tried, but again without significant long-term benefit.
If a cat with FIP is only showing mild signs of illness, the disease does not seem to be progressing, and the animal is maintaining weight and activity, everything possible should be done to support it medically. This includes rest, no stressful activities or procedures, and a high-protein diet. Even with such support, most cats with clinical FIP will eventually succumb; however, if any animal with FIP has a chance to build up enough immunity and turn the disease around, it is the cat that is still maintaining weight and activity. In contrast, supportive treatment of cats with severe, progressive, and debilitating disease is difficult to justify given the grim prognosis and the suffering that the animal will have to endure.
The Nature of Feline Coronavirus Immunity
Immunity to FECV Infection
FECV replication and clinical signs of illness are terminated with the advent of specific humoral (antibody) immunity. Humoral immunity is generated when FECV particles are carried to the regional lymph nodes, where they stimulate specific immune cells. Although detectable levels of antibody take from 7 to 14 days to appear in the serum, biologically significant levels of antibody are probably attained within a few days.
Antibodies will ultimately be secreted into the mucus film overlying the intestinal epithelium. These secretory antibodies are of the IgA class and will bind to the surfaces of free virus particles that have been released from infected cells. This binding will interfere with the ability of the virus to attach to the surfaces of new enterocytes. This blocking process will halt the infection of any new cells. Virus that is already within enterocytes is not affected by antibodies, because antibodies cannot penetrate into the cytoplasm; however, the cells at the tips of the intestinal villi are short-lived (a day or so) and will quickly die of old age and be replaced by new noninfected ones.
Although cats infected with FECV mount a vigorous and protective antibody response (humoral immunity), they do not seem to develop significant levels of cell-mediated immunity. The obvious explanation is that cell-mediated immunity is not required in FECV infection. Furthermore, the generation of cell-mediated immunity usually requires that the pathogen take up residence and replicate within cells of the immune system, in particular macrophages. FECV apparently does not replicate in macrophages, but only in the epithelial cells of the bowel.
Immunity to FIPV Infection
The fate of the newly FIPV-infected cat rests with the ability of the immune system to rapidly muster a specific attack on the infected macrophages and the virus contained within them. Because the virus particles are replicating within macrophages, they are not accessible to antibodies. Therefore, "cell-mediated immunity", and not "antibody-mediated" immunity, is the only effective means that the body has to fight the infection.
Cell-mediated immunity has two arms, both of which are triggered by anti-FIPV-specific T lymphocytes. These anti-FIPV-specific T cells, which are present in very low numbers in previously unexposed cats, constantly survey the body for signs of FIPV-infected cells. When an infected cell is recognized, the FIPV-specific T cell begins to proliferate in order to expand its numbers to cope with the rapidly escalating viral replication. It is the progeny of these first FIPV-specific T lymphocytes that will mediate the two-armed attack on the virus. The first arm involves the ability of the macrophages, upon appropriate stimuli from anti-FIPV-specific T lymphocytes, to alter their metabolic functions and to destroy the FIPV contained within their cytoplasm. The second arm is mediated by anti-FIPV-specific T cells that recognize, briefly touch, and, in the process, kill virus-infected macrophages, thus preventing viral replication. Although specific FIPV immunity begins to develop within moments of initial infection, it takes several days to a week or more before the immunity has reached sufficient intensity to halt the infection. Therefore, the outcome of the battle between the host cat and the invading virus is determined within the first few days of the infection.
Infection and FIP Immunity
Circumstantial evidence indicates that the outcome of FIPV infection is determined by the type and strength of this initial immune response, in particular the cell-mediated immunity (Pedersen, 1987). Cats that respond quickly to the infection with strong cell-mediated immunity will rapidly contain the virus within the lymph nodes. Following containment, the virus is either eliminated or allowed to persist in low numbers within a rare macrophage in the mesenteric lymph nodes or serosal surfaces. For some cats, this residue of infection may persist for a lifetime, and in a small proportion, may serve as a nidus for recrudescent disease later in life.
Role of Host Immunity in Effusive and Noneffusive FIP
Without cell-mediated immunity, FIPV replication is largely unchecked. The cycle of macrophage infection, macrophage death and release of virus, and the uptake of virus by more macrophages becomes more and more widespread and massive, resulting in a disease state referred to as "effusive FIP."
The hallmark of effusive FIP is the presence of numerous microscopic accumulations of infected macrophages around venules within the serosal/pleural surfaces and omentum. Heavily infected and diseased macrophages within perivascular pyogranulomas elaborate numerous inflammatory substances, called cytokines, into the surrounding tissues. The effect of these substances is to cause a strange type of inflammation centered around venules. This vasculitis leads to an outpouring of plasma from the blood into the body cavity. The fluid is rich in clotting factors, which have been activated in an attempt by the body to stop the vascular leakage. The yellowish color of the fluid is a byproduct of the destruction of red blood cells by phagocytic cells after they have leaked from damaged venules (the reddish hemoglobin is broken down into bilirubin, which has a yellowish color).
Between these opposite extremes of strong and weak cell-mediated immunity is an intermediate state of disease or health, depending on your viewpoint of "the glass being half full or half empty". Some cats, although not able to mount a fully effective cell-mediated immunity, are able to respond sufficiently to partially contain, but not eliminate, the virus. Such cats will develop the noneffusive or dry form of FIP (Pedersen, 1987). The lesions of noneffusive FIP are more classical granulomas made up of a core of macrophages and a surrounding mantle of lymphocytes and plasma cells. This dense mantle of lymphocytes and plasma cells is not seen surrounding the pyogranulomas that characterize effusive FIP. The presence of lymphocytes and plasma cells indicates that there is some sort of immunity in force, because these are the effector cells of immunity. Unlike the virus-laden macrophages found in effusive FIP, many of the macrophages in lesions of noneffusive FIP contain either no virus or a small amount of virus. This paucity of virus within macrophages of cats with dry FIP suggests that immunity is partially effective, i.e., it can slow down and alter the tempo of the virus replication, but not terminate it.
Antibody-Dependent Enhancement of FIP
A great deal has been made of the phenomenon of antibody-dependent enhancement of disease in FIPV infection. This phenomenon was first reported a decade and a half ago (Pedersen and Boyle, 1980; Weiss and Scott, 1981) and its importance has recently resurfaced with the introduction of live FIPV vaccines (Addie et al, 1995; Fehr et at, 1995; Gerber, 1995; McArdle et al, 1995; Scott, 1995). Antibody-mediated enhancement can be produced by infecting cats first with an FECV type FCoV (which elicits antibodies but not cellular immunity) and then challenge-expose them later with an FIPV type FCoV. Instead of getting sick with FIPV after 14 to 21 days and living for several weeks or more, which is the normal course of FIPV infection in coronavirus naive cats, FECV pre-immunized cats become sick within 24 to 72 hours and are dead within a week or two (Pedersen and Boyle, 1984). This phenomenon has been likened to the dengue hemorrhagic shock syndrome that is seen with dengue fever virus infection of humans (Weiss and Scott, 1981). However, unlike dengue hemorrhagic shock syndrome, there is no evidence that accelerated FIP occurs among cats in nature (Addie et at, 1995; Fehr et al, 1995).
The mechanisms behind antibody-dependent enhancement in FIP have been well documented. Antibodies to FECV will bind to FIPV in the same manner, but instead of preventing the virus from attaching to macrophages, the antibody enhances the uptake of virus by these cells (Stoddart and Scott, 1989). Although macrophages may lack the FCoV-specific viral receptors present on gut cells, immune complexes of FIPV and antibody can be taken up by macrophages through what are called Fc, or complement binding, receptors. Complement protein will be bound to aggregates of virus and antibody forming immune complexes; these complexes are then bound to macrophages through the Fc receptors. This uptake of FIPV containing immune complexes by macrophages has a disastrous effect, because it places the virus in the very cell where it prefers to grow. Enhanced uptake of the virus coated with antibody complement by macrophages causes both a more rapid proliferation of virus and an increased dissemination of virus to other sites in the body.
Virus-antibody-complement complexes play other roles in accelerated FIP (Jabobse-Geels et al, 1980). The virus-antibody-complement complexes are concentrated in the same areas as the infected macrophages, i.e., around small blood vessels in the target tissues. The inflammatory response that occurs in response to these complexes, as well as to the cytokines released by infected macrophages, have a damaging effect on these small blood vessels, causing them to leak tremendous amounts of fluid from the blood. It is this fluid that is characteristic of wet FIP.
Prevention of Feline Coronavirus Infections
Husbandry
It is becoming increasingly evident that prevention of FIP should be directed at prevention of the underlying FECV infections. FECV infection is a fecal-oral pathogen. Like all microbial pathogens that are transmitted in the feces and infectious by mouth, proper husbandry is essential. Husbandry must be directed at preventing fecal contamination of the environment and preventing susceptible animals from coming into contact with fecal contaminated food, water, litter, floors, etc. Proper husbandry involves two general objectives:
- construction of facilities that will allow for proper husbandry of the number and types (breeders, non-breeders, kittens, adults) of cats that are desired, and
- maintenance of procedures within those facilities that will minimize fecal-oral contact, especially between cats that shed the virus and susceptible animals.
Means to accomplish both of these objectives have been described (Pedersen and Wastlhuber, 1991).
FCoV infection can be eliminated from catteries by proper husbandry procedures (Addie and Jarrett, 1995; Hickman et al, 1995). Eradication is made possible by two characteristics of the infection:
- most infected cats do not shed the virus for long periods of time (Addie and Jarrett, 1995; Hickman et al, 1995), and
- kittens born to exposed queens seem to be protected by maternal immunity for the first 6 weeks or more of life (Pedersen, 1995).
Therefore, if time is allowed for infected animals to stop shedding, and new reinfections are prevented, the virus can be eliminated from a premise.
Eradication of FCoV infection is best accomplished by isolating all pregnant queens in separate pathogen-free quarters and weaning the kittens before their maternal immunity wanes (Addie and Jarrett, 1995). Early weaning, usually at less than 6 weeks of age, is necessary only in situations where the queen herself may be shedding the virus. If queens are not shedding FCoV, weaning can be delayed until whenever it is most appropriate. The likelihood that a queen is shedding virus appears to be proportional to her FCoV antibody titer. Queens with titers of below 1:25 may not shed virus, while queens with titers of 1:100 or above are frequently infectious (Addie and Jarrett, 1995; Hickman et al, 1995; Pedersen et al, 1981). Queens older than two years of age are also much less likely to infect their kittens than younger queens (Kass and Dent, 1995).
Following weaning, the queen is removed and the kittens are reared as a single litter in isolation from other cats until they are 12 to 16 weeks of age. At that time, they are tested for FCoV antibodies. It they are negative at this age, no exposure occurred either from the mother or from inadvertent contact with virus from outside the isolation quarters. If they are antibody positive, the isolation procedure failed.
Although isolation of queens and controlled weaning has been successful in eradicating FCoV infection from groups of cats, the success of the whole program depends on the ability to prevent inadvertent spread of the virus between isolated cats and cats that are not isolated in the same environment (Hickman et al, 1995). Such containment requires proper isolation quarters and precise husbandry techniques (Hickman et al, 1995); FCoVs are easily spread between one group of cats and another on human clothing (Pedersen et al, 1981) and in even the most careful of quarantine situations (Hickman et al, 1995). The greater the number of animals, and the less sophisticated the cattery design, the more likely the eradication will fail. In fact, it is highly unlikely that such an eradication program can succeed or be sustained in catteries run out of human homes, especially if more than 8 to 10 breeding cats are involved. Moreover, once the virus is eradicated, extreme steps must be taken in keeping it from reentering. Such steps may even preclude normal cattery activities such as bringing in new animals.
Eradication of FCoV infection could be greatly simplified with the development of specific tests to detect virus shedders. Serology, while giving some idea as to which cats may be shedding virus and which are not, is not accurate enough for such a purpose. Therefore, tests to detect FCoVs in feces are badly needed. At the present time, the PCR technique holds the greatest promise (Herrewegh et al, 1995). This procedure is still very expensive and requires a level of sophistication that is above most commercial diagnostic laboratories.
Vaccination
The ideal vaccine for FIP has been described as a live FIPV-based vaccine that would be sufficiently invasive of macrophages to induce cell-mediated immunity without actually causing the disease that it is meant to prevent (Pedersen, 1989; Pedersen and Black, 1983). Feline enteric coronavirus-based vaccines will not protect cats against FIPV because they elicit antibody and not cell-mediated immunity. This type of immunity will result in enhanced disease rather than protection against FIPV (Pedersen and Boyle, 1980). A live FIPV-based vaccine that meets many of these criteria has been recently marketed for the prevention of FIP (Primucell FIP™, Plizer Animal Health, Inc., Exton, PA). Preliminary reports on this vaccine have been provided elsewhere in this issue (Fehr et al, 1995; Gerber, 1995; Hoskins et al, 1995a; McArdle et al, 1995; Postorino Reeves, 1995; Scott, 1995a).
Preliminary studies indicate that Primucell FIP vaccine is safe; however, there have been experimental differences on whether the vaccine can induce antibody-dependent enhancement. Some groups report enhancement in cats with this product (McArdle et al, 1995; Scott, 1995b), while others have not observed such a problem (Gerber, 1995; Hoskins, 1995a). Differences in the various experimental findings can best be explained by the strain of FIPV used for the challenge-exposure. Serotype II strains such as FIPV-79-1146 (and presumably the genetically identical FIPV-DF2 and FIPV-Norl5) do not cause accelerated disease when used as the challenge strain in healthy FCoV antibody positive cats (Pedersen et al, 1984). Therefore, these challenge-exposure strains are not appropriate for evaluating antibody dependent enhancement. Although these various studies differ on the ability of this vaccine to evoke antibody-dependent enhancement of FIPV infection, the issue of vaccine-induced disease enhancement may be a moot point. Antibody-dependent enhancement, although a real phenomenon in experimental studies, does not seem to occur in nature (Addie et al, 1995; Fehr et al, 1995).
The efficacy of Primucell FIP has been conclusively demonstrated for cats that are at least 16 weeks of age, and FCoV antibody negative, at the time of vaccination. Under such conditions, efficacy has ranged from 50% (Hoskins et al, 1995a) to 75% (Postorino Reeves, 1995) or more (Gerber, 1995). Although Primucell FIP is efficacious in FCoV seronegative cats 16 weeks of age or older, its efficacy has not been reported for younger cats or FCoV seropositive cats. However, Gerber (Pfizer, unpublished observation, 1994) stated that efficacy against experimental FIPV challenge-exposure is much less in very young kittens. Efficacy also appears to be negligible in the field when used on seropositive animals coming from highly FCoV endemic households (Fehr et al, 1995). The poor efficacy in seropositive cats in the field could be because of either interference by acquired FCoV immunity or the fact that most cats in multiple-cat households have already been exposed by 16 weeks of age and vaccination cannot alter the subsequent disease course (Fehr et al, 1995).
The efficacy of Primucell FIP vaccine against a wide variety of field strains of FIPV has not been determined. The virulence-attenuated vaccine virus was derived from a Serotype II FIPV and therefore would be expected to induce maximum protection against field isolates of the same serotype. Most studies that have shown protection have involved challenge-exposure with FIPV-DF2, FIPV-Norl5 or FIPV-79-1146 strains. All of these strains are very closely related to the vaccine strain but are antigenically different from the predominant Serotype I strains of FCoV that exist in the field. Therefore, it is possible that Primucell FIP would be less efficacious against Serotype I FCoVs. McCardle and associates (1995) challenged vaccinated cats with a field strain from the United Kingdom and were unable to demonstrate protection. Unfortunately, they did not determine the serotype of their challenge virus. Fehr and colleagues (1995) and Postorino Reeves (1995) in field studies in Switzerland and the U.S., respectively, observed some protection, indicating that Primucell FIP vaccine virus is cross-reactive with a proportion of natural strains.
If vaccines such as Primucell FIP have 50 to 75% efficacy when given to FCoV seronegative cats 16 weeks of age or older, how should they be used in the field? First, until the issues concerning their efficacy in previously FCoV exposed cats can be resolved, they cannot be highly recommended for use in cats, 16 weeks or older, coming from environments likely to be endemic with FCoVs. What about the use of vaccine in groups of cats likely to be FCoV seronegative, such as cats from households with one, two or three animals? While experimental evidence suggests that this group will develop the best immunity from vaccination, the incidence of FIP in such pet cats is on the order of one case per 5,000 cats. Vaccination under such circumstances would not be nearly as efficient in decreasing overall FIP as would immunization of cats from high risk environments.
The use of Primucell FIP on 6-week-old kittens has been described by Panzero (1992) and this practice has become increasingly popular with cat breeders. Vaccination at a young age was reportedly successful in stopping an outbreak of FIP in a cattery of Cornish Rex cats. However, outbreaks of FIP often wax and wane on their own, so it is not possible to say that the vaccine was responsible for the subsequent drop in FIP deaths. Nevertheless, this type of vaccination regimen has some rationale. Kittens lose their maternal antibody at about this time and should be responsive to vaccination. If the vaccine virus were to induce anti-FCoV humoral immunity, subsequent infection with endemic FECVs might be prevented. If the mutational theory regarding the origin of FIPV was correct, preventing FECV infection might be effective in preventing mutant forms of the virus from appearing. Hoskins and colleagues (1995) have vaccinated young SPF kittens with Primucell FIP vaccine and challenged them experimentally with FECV-79-1683. He was able to show a significant reduction in virus shedding in vaccinated versus unvaccinated kittens. On a negative note, various strains of coronaviruses may genetically recombine with each other in dual infections (Horzinek et al, 1995), and vaccine strains of infectious bronchitis coronavirus of chickens have been shown to recombine with wild type virus and form new virulent strains (Kusters et al, 1990). Therefore, until the efficacy and safety of early vaccination can be proven, the use of Primucell FIP in very young kittens in FCoV endemic households cannot be unconditionally supported.
References
- Addie D and Jarrett O. Control of feline coronavirus infection in breeding catteries by serotesting, isolation and early weening. Feline Practice in press, 1955.
- Addie D, Toth S, Murray GD and Jarrett O. The risk of typical and antibody enhanced feline infectious peritonitis among cats from feline coronavirus endemic households in the United Kingdom. Feline Practice in press, 1995.
- Boyle JE, et al. Plaque assay, polypeptide composition and immunochemistry of feline infectious peritonitis virus and feline enteric coronavirus. Adv Exp Med Biol 173: 133-147, 1984.
- Cotter SM, et al. Multiple cases of feline leukemia and feline infectious peritonitis in a household. JAVMA 162: 1054-1058, 1973.
- Dea D, et al. Coronavirus-like particles in the feces of a cat with diarrhea. Can Vet J 232: 153-155, 1982.
- Fehr D, Holznagel E, Bolla S, Hauser B, Herrewegh AAPM, Horzinek M and Lutz H. Evaluation of the safety and efficacy of a modified live FIPV vaccine under field conditions. Feline Practice in press, 1995.
- Gerber J. Overview of the development of a modified live temperature sensitive FIP virus vaccine. Feline Practice in press, 1995.
- Herrewegh A. Polymerase chain reaction (PCR) for diagnosis of naturally occurring feline coronavirus infections. Feline Practice in press, 1995.
- Hickman A, Morris JG, Rogers QR and Pedersen NC. Elimination of feline coronavirus infection from a large experimental specific pathogen free cat breeding colony by serologic testing and isolation. Feline Practice in press, 1995.
- Hohdatsu T, Okada S, et at. The prevalence of types I and II feline coronavirus infections in cats. J Vet Med Sci 54: 557-562, 1992.
- Holmberg CA and Gribble DH. Feline infectious peritonitis: Diagnostic gross and microscopic lesions. Feline Practice 3(4): 11-14, 1973.
- Horzinek M, Herrewegh A and de Groot R. Perspectives on feline coronavirus evoluation. Feline Practice in press, 1995.
- Hoskins JD, Taylor HW and Lomax TL. Independent evaluation of a modified live FIPV vaccine under experimental conditions (Louisiana State University experience). Feline Practice in press, 1995a.
- Hoskins JD, Henk WG, Storz J and Kearney MT. The potential use of a modified live FIPV vaccine to prevent experimental FECV infection. Feline Practice in press, 1995.
- Jacobse-Geels HEL, et al. Isolation and characterization of feline C3 and evidence for the immune complex pathogenesis of feline infectious peritonitis. J Immunol 125: 1606-1610, 1980.
- Kass P and Dent T. The epidemiology of FIP in catteries. Feline Practice in press, 1995.
- Kusters JG, Jager EJ. et al. Sequence evidence for RNA recombination in field isolates of avian coronavirus infectious bronchitus virus. Vaccine 8: 605-608, 1990.
- Martinez ML and Weiss RC. Detection of feline infectious peritonitis virus infection in cell cultures and peripheral blood mononuclear leukocytes of experimentally infected cats using a biotinylated cDNA probe. Vet Micro 34: 259-271, 1993.
- McArdle F, Tennant B, Kelly DF, Gaskell CJ and Gaskell RM. Independent evaluation of a modified live FIPV vaccine under experimental conditions (Univesity of Liverpool experience). Feline Practice in press, 1995.
- McKiernan AJ, et al. Isolation of feline coronaviruses from two cats with diverse disease manifestations. Feline Practice 11(3): 16-20, 1981.
- Montali RJ and Strandberg JD. Extraperitoneal lesions in feline infectious peritonitis. Vet Path 9: 109-121, 1972.
- Panzero R. An outbreak of feline infectious peritonitis in a colony of Cornish Rex cats. Feline Practice 20(4): 7-8, 1992.
- Pedersen NC. Feline infectious peritonitis. Something old, something new. Feline Practice 6(3): 42-51, 1976a.
- Pedersen NC. Serologic studies of naturally occurring feline infectious peritonitis. AJVR 37: 1449-1453, 1976b.
- Pedersen NC. Feline infectious peritonitis test results: What do they mean? Feline Practice 7(3): 13-14, 1977.
- Pedersen NC. Feline infectious peritonitis and feline enteric coronavirus infections. Part I: Feline enteric coronavirus. Feline Practice 13(4): 13-19, 1983.
- Pedersen NC. Feline infectious peritonitis and feline enteric coronavirus infections. Part II: Feline infectious peritonitis. Feline Practice 13(5): 5-19, 1983.
- Pedersen NC. Virologic and immunologic aspects of feline infectious peritonitis virsis infection. Adv Exp Biol Med 218:529-550, 1987.
- Pedersen NC. Animal virus infections that defy vaccination: Equine infectious anemia, caprine arthritis-encephalitis, maedi-visna, and feline infectious peritonitis. Adv Vet Sci Comp Med 33:413-428, 1989.
- Pedersen NC. The history and interpretation of feline coronavirus serology. Feline Practice in press, 1995.
- Pedersen NC and Black JW. Attempted immunization of cats against feline infectious peritonitis using either avirulent live virus or sublethal amounts of virulent virus. AJVR 44:229-234, 1983.
- Pedersen NC and Boyle JF. Immunologic phenomena in the effusive form of feline infectious peritonotis. AJVR 41:868-876, 1980.
- Pedersen NC, Black JW, et al. Pathogenic differences between varius feline coronavirus isolates. In Coronaviruses; molecular biology and pathogenesis. Adv Exp Med Biol 173:365-380, 1984.
- Pedersen NC, Boyle JF, et at. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. AJVR 42:368-377, 1981.
- Pedersen NC, Evermann JF, et al. Pathogenicity studies of feline coronavirus isolates 79-1146 and 79-1683. AJVR 45:2580-2585, 1984.
- Pedersen NC and Floyd K. Experimental studies with three new strains of feline infectious peritonitis virus FIPV-UCD2, FIPV-UCD3, and FIPV-UCD4. Comp Cont Edu Pract Vet 7:1001-1011, 1985.
- Pedersen NC, Ward J, et al. Antigenic relationship of the feline infectious peritonitis virus to coronaviruses of other species. Arch Virol 58:45-53, 1978.
- Pedersen NC and Wastlhuber J. Cattery design and management. In: Pedersen NC, Feline Husbandry, American Veterinary Publications (Mosby), Goleta, CA, 1991, pp 393-446.
- Postorino Reeves N. Vaccination against naturally occcuring FIP in a single large cat shelter. Feline Practice in press, 1995.
- Richards JR. Problems in the interpretation of feline coronavirus serology (specificity vs. sensitivity). Feline Practice in press, 1995
- Scott F. Independent evaluation of modified live FIPV vaccine under experimental conditions (Cornell University experience). Feline Practice in press. 1995a.
- Scott F. The phenomenon of antibody mediated enhancement of experimentally induced FIP and its implication to vaccination in the field. Feline Practice in press, 1995b.
- Stoddart CA and Scott FW. lntrinsic resistance of feline peritoneal macrophages to coronaviruses correlates with virulence. J Virol 63: 436-440, 1989.
- Vennema H, Poland A, Floyd Hawkins K and Pedersen NC. A comparison of the genomes of FECVs and FIPVs: What they tell us about the relationships between feline coronaviruses and their evolution. Feline Practice in press, 1995.
- Vennema H, et at. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization, J Virol 64: 1407-1409, 1990.
- Ward JM. Morphogenesis of a virus in cats with experimental feline infectious peritonitis. Virol. 41: 191-194, 1970.
- Weiss R and Scott F. Antibody-mediated enhancement of disease in feline infectious peritonitis: Comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis 4: 175-189, 1981.
- Weiss R. The state of knowledge on the treatment of FIP with antiviral drugs or immune response modifiers. Feline Practice in press, 1995.
- Wolf J. The impact of feline infectious peritonitis on catteries. Feline Practice in press, 1995.
- Wolfe LG and Griesemer RA. Feline infectious peritonitis. Path Vet 3: 255-270, 1966.
- Zook BC et al. Ulrastructural evidence for the viral etiology of feline infectious peritonitis. Path Vet 5: 91-95, 196.
BAER Test Cristalline
By Daniëlle Rozeboom, Traditional Persian Cattery Yeri Shaes.
(Reprinted with permission)
In The Netherlands it's not allowed to breed with deaf white cats (also not cats who are deaf on only one ear). To prove that a white kitty is not deaf, you need to do the BAER test. In The Netherlands there are not many locations where you can do the BAER test. Luckily there is one not too far away from where we live. Today we took Cristalline to the clinic of dr Dijkshoorn in Zeist.
The hearing test known as the brainstem auditory evoked response (BAER) or brainstem auditory evoked potential (BAEP) detects electrical activity in the cochlea and auditory pathways in the brain in much the same way that an antenna detects radio or TV signals or an EKG detects electrical activity of the heart.

On our way in the car. She definitely did not like it in the car,
she wanted to go out! She stayed in my arms for a while,
but it's safer in the travel kennel.

You have to bring the pedigree with you.
At the clinic Cristalline got first an injection with
anesthetics. She slowly fell asleep in my arms.

Sweet dreams...

The response is collected with a special computer
through extremely small electrodes placed under the
skin of the scalp: one in front of each ear,
one at the top of the head.
The stimulus click produced by the computer is directed
into the ear with a foam insert earphone.
Here placed in the left ear.

It's very clear Cristalline can hear with her left ear!
No doubt about it :-) One to go...

The other ear is tested now...

Yeah! Even better hearing. Cristalline is not deaf!
Ahh... poor Cristalline...

Time to wake up now...

What just happened?

On our way home...
© Daniëlle Rozeboom, March 14th 2008.
